INDIAN ARMED FORCES CHIEFS ON
OUR RELENTLESS AND FOCUSED PUBLISHING EFFORTS

SP Guide Publications puts forth a well compiled articulation of issues, pursuits and accomplishments of the Indian Army, over the years

I am confident that SP Guide Publications would continue to inform, inspire and influence.

My compliments to SP Guide Publications for informative and credible reportage on contemporary aerospace issues over the past six decades.
DRDO’s anti-COVID drug, a silver lining
The Drug Controller General of India has granted permission for emergency use of the drug as adjunct therapy in moderate to severe COVID-19 patients.
As the country deals with the challenge and chaos caused due to the second wave of COVID-19, a ray of hope seems to be appearing. The Institute of Nuclear Medicine and Allied Sciences (INMAS), a lab of Defence Research and Development Organisation (DRDO), has developed an anti-COVID-19 therapeutic application of the drug 2-deoxy-D-glucose (2-DG) in collaboration with Dr Reddy’s Laboratories (DRL), Hyderabad. The clinical trial results of the drug have displayed that this molecule assists in faster recovery of hospitalised patients and reduces supplemental oxygen dependence, which indeed is a significant development, considering the panic caused due to the lack of oxygen cylinders in various parts of the nation. Hundreds of deaths have been reported over the last month due to patients’ dropping oxygen levels, social media too has been filled with requests for leads on oxygen cylinders. Amid such scenario, having an indigenous drug that can counter oxygen dependence can prove to be a seriously needed boon.
The clinical trial results of the drug have displayed that this molecule assists in faster recovery of hospitalised patients and reduces supplemental oxygen dependence.
As per the information released by the Ministry of Defence (MOD), Government of India, higher proportion of patients treated with 2-DG have showed RT-PCR negative conversion in COVID patients.
An anti-COVID-19 therapeutic application of the drug 2-deoxy-D-glucose (2-DG) has been developed by INMAS, a lab of DRDO, in collaboration with Dr Reddy’s Laboratories, Hyderabad. The drug will help in faster recovery of Covid-19 patients. https://t.co/HBKdAnZCCP pic.twitter.com/8D6TDdcoI7
— DRDO (@DRDO_India) May 8, 2021
It was last year in April 2020, when the first wave of the pandemic had hit the country, that INMAS-DRDO scientists conducted laboratory experiments with the help of Centre for Cellular and Molecular Biology (CCMB), Hyderabad. The experiments conducted at that time had revealed that the effective working of this molecule against SARS-CoV-2 virus and inhibits the viral growth. Based on these results, phase-II clinical trial of 2-DG in COVID-19 patients were permitted in May 2020 by Drugs Controller General of India’s (DCGI) Central Drugs Standard Control Organisation (CDSCO).
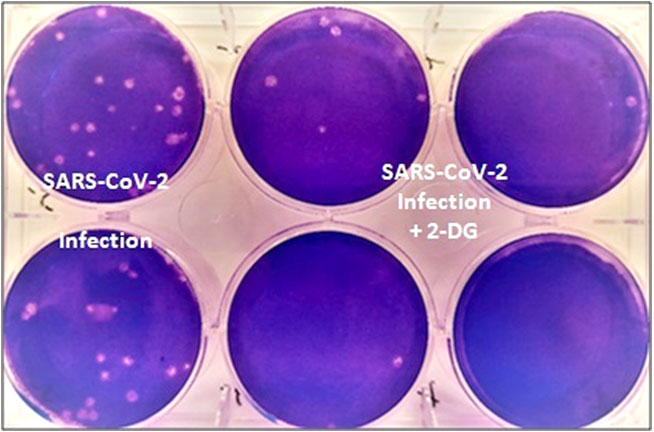
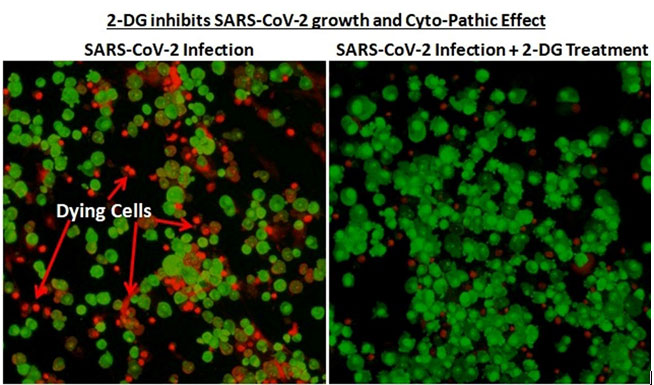
Trials
The DRDO, along with its industry partner DRL, Hyderabad initiated the clinical trials to test the safety and efficacy of the drug in COVID-19 patients. In phase-II trials (including dose ranging) that were conducted last year from May to October 2020, the drug was found to be safe in COVID-19 patients and even showed significant improvement in their recovery.
The phase–II clinical trial was further branched into two phases. Phase-II a was conducted in six hospitals and Phase IIb (dose ranging) clinical trial was conducted at 11 hospitals all over the country. Phase-II trial was conducted on 110 patients, MOD’s statement informed.
It accumulates in the virus infected cells and prevents virus growth by stopping viral synthesis and energy production. Its selective accumulation in virally infected cells makes this drug unique.
It further added that while observing the efficacy trends, the patients treated with 2-DG showed faster symptomatic cure than Standard of Care (SoC) on various endpoints. A significantly favourable trend (with a difference of 2.5 days) was noticed in terms of the median time to achieving normalisation of specific vital signs parameters when compared to SoC.
Based on successful results, DCGI gave the permission for the Phase-III clinical trials in November 2020. The Phase-III clinical trial was then carried on 220 patients between December 2020 to March 2021 at 27 COVID hospitals in Delhi, Uttar Pradesh, West Bengal, Gujarat, Rajasthan, Maharashtra, Andhra Pradesh, Telangana, Karnataka and Tamil Nadu. The detailed data of phase-III clinical trial was then presented to DCGI. According to the statement, “In 2-DG arm, significantly higher proportion of patients improved symptomatically and became free from supplemental oxygen dependence (42% vs 31%)” by the third dayas against the SoC. This indicates an early relief from oxygen therapy or oxygen dependence.The similar trend was also observed in patients above 65 years of age.
Following this, on May 1, 2021, the DCGI granted permission for emergency use of this drug as adjunct therapy in moderate to severe COVID-19 patients. What is more significant to note is that MOD has stated the possibility of easy production and availability in plenty in the country, being a generic molecule and analogue of glucose.
How does it work?
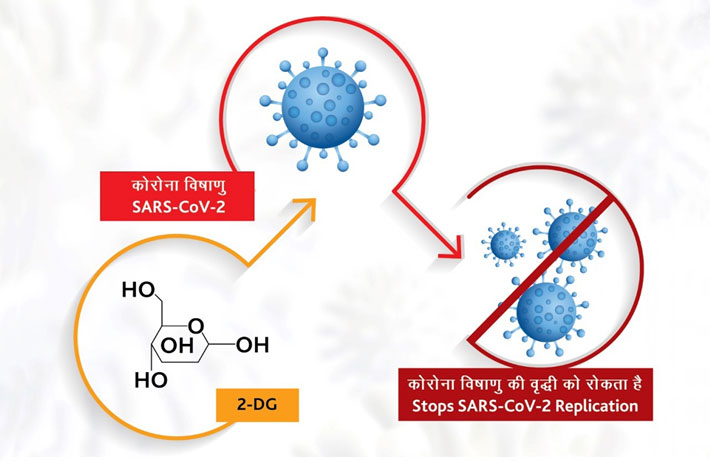
The drug comes in powder form in sachet, which is taken orally by dissolving it in water. It accumulates in the virus infected cells and prevents virus growth by stopping viral synthesis and energy production. Its selective accumulation in virally infected cells makes this drug unique. The drug is expected to save lives due to the mechanism of operation of the drug in infected cells. This also reduces the hospital stay of COVID-19 patients.
DRDO to set up 500 Medical Oxygen Plant
DRDO has been actively coming to the fore and contributing in various manners. Just at the end of last month, the Ministry had announced that the Medical Oxygen Plant (MOP) technology, developed by DRDO shall aid in fighting the current crisis of oxygen for COVID-19 patients. The technology was developed for On-Board Oxygen Generation for LCA (Light Combat Aircraft)Tejas by DRDO’s lab, DEBEL (Defence Bioengineering and Electromedical Laboratory).
In order to tackle the surge in COVID-19 cases & subsequent requirement of oxygen, PM-CARES has allocated funds for the installation of 500 Medical Oxygen Plants across the country. These plants are planned to be set up within three months. @DRDO_India pic.twitter.com/qmMmiQmlTn
— ????? ?????? ????????/ RMO India (@DefenceMinIndia) May 4, 2021
MOP technology is capable of generating oxygen with 93±3% concentration which can be directly supplied to hospital beds or can be used to fill medical oxygen cylinders. It utilises Pressure Swing Adsorption (PSA) technique and Molecular Sieve (Zeolite) technology to generate oxygen directly from atmospheric air. Hence, with the MOP technology, hospitals will be able to generate on site medical oxygen, in a cost-effective manner with this oxygen plant.

The oxygen plant is designed for a capacity of 1,000 litres per minute (LPM). The ministry informed that system can cater to 190 patients at a flow rate of 5 LPM and charge 195 cylinders per day. Transfer of Technology has been done to Tata Advanced Systems Limited, Bengaluru and Trident Pneumatics Pvt. Ltd., Coimbatore, who will be producing 380 plants for installation across various hospitals in the country. 120 plants of 500 LPM capacity will be produced by industries working with Indian Institute of Petroleum, Dehradun, belonging to CSIR (Council of Scientific and Industrial Research). The plant complies with International Standards like ISO 1008, European, US and Indian Pharmacopeia, stated the release.
MOP technology is capable of generating oxygen with 93±3% concentration which can be directly supplied to hospital beds or can be used to fill medical oxygen cylinders.
MOD informed that MOP has already been installed at some of the Army sites in North East and Leh-Ladakh region and site preparation for 5 plants to be installed in Delhi/NCR region has been initiated. Further to that, the DRDO has also begun fabrication of 380 MOPs with release of supply orders for 332 numbers on Tata Advanced Systems and 48 on Trident Pneumatics. DRDO is targeting to produce 125 plants per month under PM CARES Fund and with that, it expects that 500 MOPs will be installed within three months.
DRDO has also been working on setting up hospitals and providing for beds in various parts of the country including New Delhi, Lucknow, Varanasi with army personnel swiftly taking up to the task.
Pandit Rajan Mishra Covid Hospital set up by DRDO with support of state administration in Varanasi is being operationalised today. All the doctors and paramedical staff are from Armed Forces Medical Services. #IndiaFightsCorona pic.twitter.com/lfOHpHIu2O
— DRDO (@DRDO_India) May 10, 2021
SpO2 based Supplemental Oxygen Delivery System
Earlier last month, DRDO had also developed SpO2 (Blood Oxygen Saturation) supplemental Oxygen Delivery System its lab DEBEL. The system delivers supplemental oxygen based on the SpO2 levels and prevents the person from sinking in to a state of Hypoxia, which is said to be fatal in most cases, if sets in.
The system is indigenously developed for operation in field conditions, it is unique with its dual qualities of being robust and cheap and is already in bulk production with the industry.
Hypoxia is a state in which the amount of oxygen reaching the tissues is inadequate to fulfill all the energy requirements of the body. Developed for soldiers posted at extreme high-altitude areas, this automatic system can also prove to be a savior during the current Covid-19 situation.
How does the system work?
The system reads SpO2 levels of the subject from a wrist-worn pulse oximeter module through wireless interface and controls a proportional solenoid valve to regulate the oxygen supply to the subject. The oxygen is delivered from a lightweight portable oxygen cylinder through nasal nares. The system is available in various sizes from one litre and one kg weight with 150 litres of oxygen supply to 10 litres& 10 kg weight with 1,500 litres of oxygen supply which can sustain for 750 minutes with a continuous flow of two LPM.

The statement released by the ministry mentioned that since the system is indigenously developed for operation in field conditions, it is unique with its dual qualities of being robust and cheap and is already in bulk production with the industry. “The automatic usage has huge advantage in the household, as the oximeter would give an alarm for lower SpO2 value. It will automatically increase/decrease the O2 flow based on SpO2 setting which can be auto adjusted at 2, 5, 7, 10 lpm flow rate. The optimal O2 flow rate conserves the O2 resources/O2 management and greatly increases the endurance,” the statement noted.
DRDO, is also working along with the Centre for Artificial Intelligence and Robotics (CAIR) on a program that could test patients for coronavirus much faster.
The several initiatives and steps that the DRDO is continuously taking at the time of the present crisis, clearly demonstrate the importance of research as well as the resilient and innovation spirit of our scientists during these challenging times. This is indeed in line with the National Technology day, marked on May 11 and reiterates the statement of Prime Minister Narendra Modi. “In any challenging situation, our scientists and innovators have always risen to the occasion and worked to mitigate the challenge. Over the last year, they have worked industriously to fight COVID-19. I appreciate their spirit and remarkable zeal,” the PM had tweeted.
On National Technology Day, we salute the hardwork and tenacity of our scientists and those passionate about technology. We remember with pride the 1998 Pokhran Tests, which demonstrated India’s scientific and technological prowess.
— Narendra Modi (@narendramodi) May 11, 2021





